Digital enhancement of cryoEM photographs of protein nanocry
Source: Union of Crystallography
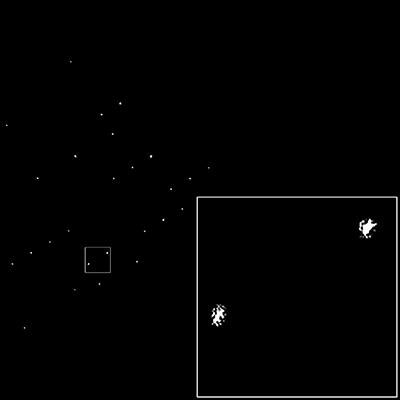
Unique Half Of The Centrosymmetric Lattice Filter Of The Lysozyme Crystal
The spots that make up the lattice of the real image are clearly visible. Because of mosaicity and crystal shape, the spots are not uniform in shape.
The procedure described by van Genderen et al. ("Lattice filter for processing image data of three-dimensional protein nanocrystals") paves the way towards full three-dimensional structure determination at high resolution for protein crystals.
When cryoEM images are obtained from protein nanocrystals the images themselves can appear to be devoid of any contrast. A group of scientists from the Netherlands have now demonstrated that lattice information can be revealed and enhanced by a specialized filter.
The authors report on how lattice information can be enhanced by means of a wave finder in combination with Wiener-type maximum-likelihood filtering. The lattice filter is a very powerful tool for selecting and analysing extremely low contrast cryo-images of three dimensional protein/peptide nanocrystals. It confirms that the three-dimensional crystals are made up from multiple domains that are slightly differently oriented. Indeed, the algorithm can comfortably deal with multiple crystals with very different orientations, unit cells and/or space groups.
The authors of the paper propose the new lattice filter as a powerful tool for processing very noisy images with crystal factors (and thus the phase information) hidden within them. The filter is able to discriminate between noise images and the very noisy images with very low contrast which contain crystal-like structures. The lattice filter retains the shape of the spots in Fourier space and also retains any phase gradients within the Bragg spots (which determine the domain structure within the crystal). Thus, it retains all of the significant information from the Bragg spots.
This will open the way to combining the phases acquired from stationary, two-dimensional images with intensities of rotation diffraction data taken from the same type of crystals. In this way, the authors expect to be able to phase the diffraction information of protein and peptide crystals.
| }
|